By Bryan G. Hopkins, Ph.D., CPSS
“Get the nitrogen right.” This is good advice. Sitting next to root zone water and oxygen management, there isn’t much else that rises to importance of nitrogen management for healthy grass.
There are 17 essential nutrients. But nitrogen has, by far, the greatest impact on grass health in most soils. The other nutrients are important too, but mismanaging nitrogen generally has larger downsides than all of the rest.
There are hundreds of nitrogen fertilizer products in the marketplace. How does one choose which products are best? This question is more easily answered once a few basics are understood.
Nitrogen cycle
The nitrogen cycle is a fundamental piece of knowledge every grass manager needs to understand (Fig. 1). It seems complex, but sports field managers need to understand a few basics of this cycle. The following are among the important keys for nitrogen fertilizer management:
- Hydrolysis converts urea to ammonia gas
- Ammonia gas can be:
- Volatilized (lost to atmosphere)
- Converted to ammonium (captured by soil)
- Ammonium is dissolved in soil water and can be:
- Attached to soil CEC colloids (clay and organic matter)
- Leached below the root zone (only in very sandy/silty soils with little organic matter)
- Taken up by plants
- Converted to nitrate in the nitrification process
- Nitrate is dissolved in soil water and can be:
- Leached below the root zone (occurs in all soils if excess water moves through)
- Converted to nitrous oxide gas and lost to the atmosphere (wet/compact soils)
- Taken up by plants
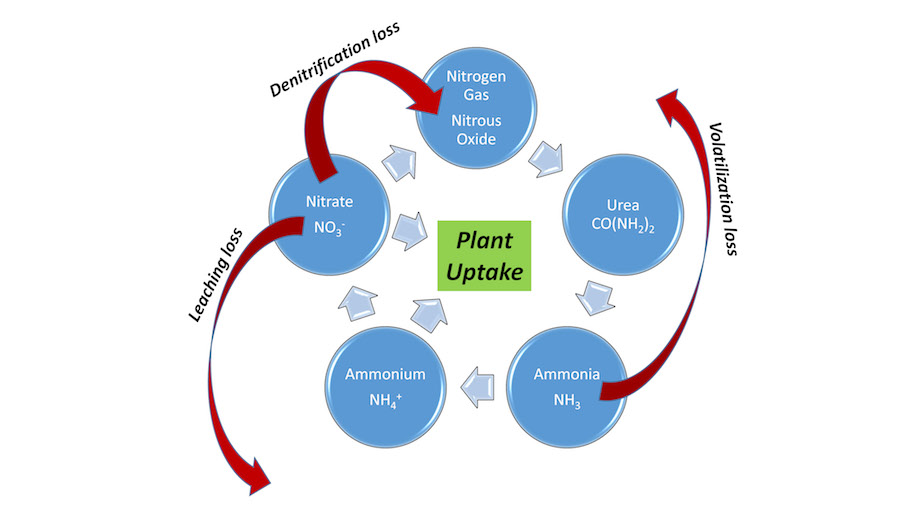
In general, nitrogen found in the soil as urea or ammonium is less at risk of loss than nitrate. Thus, it is important to get the urea and ammonium moved into the soil and slow their conversion to nitrate. Doing so results in nitrogen being more likely to be taken up by plants and not lost to the environment.
Avoid losses of nitrogen to the environment
In addition to budget concerns from nitrogen loss, grass managers should avoid loss because of the following environmental concerns:
- Wasted fertilizer wastes natural resources, such as natural gas;
- High nitrates in drinking water are a human health concern;
- Nitrogen in surface waters causes algal blooms and resultant eutrophication;
- Ammonia gas gets deposited back onto land where it can be destructive to sensitive ecosystems and contribute to eutrophication in surface waters; and
- Nitrous oxide is a potent greenhouse gas.
Some loss of nitrogen is inevitable, but research shows that managers of grass can curb most all of it. Best management practices to avoid losses to the environment include:
- Knowing which nitrogen forms (urea, ammonium, and/or nitrate or other) are found in the fertilizer and if they are in quick release or in protected form;
- Avoiding surface application of unprotected, quick release urea and ammonium based products when temperature, humidity, and/or wind are high;
- Watering urea and ammonium based products into the soil with ¼ inch of irrigation water shortly after application, especially if temperature, humidity, and/or wind are high;
- Avoiding application of excess irrigation water to avoid leaching nitrogen out of the root zone;
- Keeping the soil well aerated;
- Avoiding spikes of nitrogen in the soil by making it steadily available to plants through the entire growing season;
- Making sure plants have ample nitrogen during the fall before they go into dormancy; and
- Accomplishing the above by using a blend of quick release and enhanced-efficiency nitrogen fertilizers.
Understanding enhanced-efficiency nitrogen fertilizers.
There are many types of enhanced-efficiency fertilizers (Fig. 2). It is important to understand what part of the nitrogen cycle (Fig. 1) is being addressed with those that are inhibitors or slow/control release (Fig. 2). Not all enhanced products are equally effective – as some manufacturers have poor quality control. But these can be very effective if the product is high quality and is used properly.
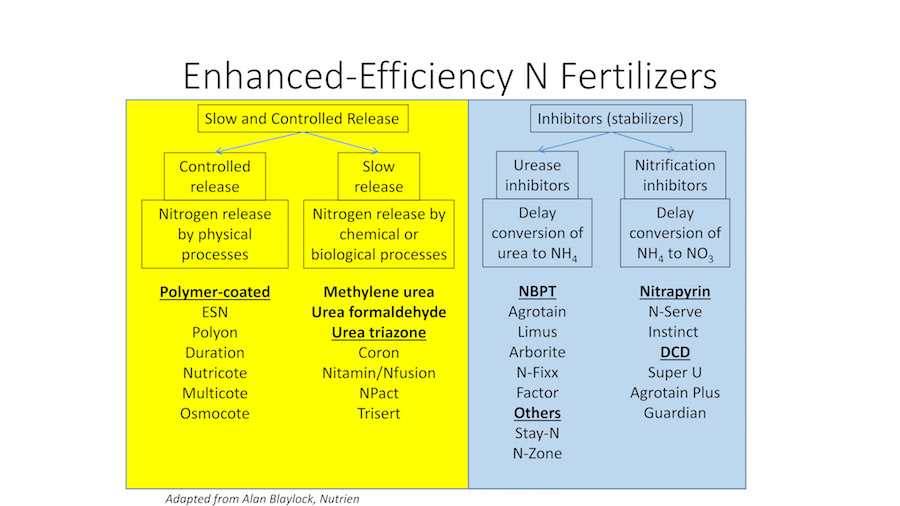
Control-releasefertilizers keep nitrogen from releasing into the soil solution until physical processes facilitate the release. For example, polymer-coated urea (see photo at top) keeps the nitrogen inside a coating until it eventually diffuses out. The nitrogen release rate increases with increasing temperatures. These are advertised with various release timings, which are relatively accurate when these are buried in soil in agricultural applications. However, when applied to the surface, the release is hastened. This occurs because grass surfaces often have temperatures in excess of 120degrees Fahrenheit. Nevertheless, these can be very effective if the coatings are not cracked through excessive handling.
Slow-releasefertilizers keep the nitrogen bound into molecules that are not taken up by plants nor lost to the environment until they break down via chemical and/or biological processes to become plant available. For example, urea-formaldehyde, methylene urea, and triazone-based fertilizers consist of long chain, nitrogen-containing molecules. Another type is sulfur-coated urea used alone or in conjunction with polymer coatings. The nitrogen becomes available to plants as the sulfur coating oxidizes over time – releasing the nitrogen into the soil. Again, the coating can become cracked and, thus, these fertilizers also need to be handled with care.
Urease inhibitors[e.g., N-butyl-thiophosphoric triamide (NBPT)] inhibit the urease enzyme, which catalyzes the hydrolysis reaction that converts urea to ammonia gas and then, if not lost to the atmosphere, to ammonium. Nitrification inhibitors[e.g., dicyandiamide (DCD), 2-chloro-6-(trichloromethyl) pyridine (nitrapyrin), N-butyl-thiophosphoric triamide (NBPT), 3,4-dimethylpyrazole phosphate (DMPP), and pronitridine] were developed to slow the conversion of ammonium to nitrate by inhibiting the activity of Nitrosomonas spp. bacteria responsible for this conversion.
Normally, urea hydrolysis to ammonium is complete within two to four days. A urease inhibitor slows it to seven to 14 days. Conversion of ammonium to nitrate normally is complete within seven to 21 days. A nitrification inhibitor slows that to 25 to 50 days. Using both inhibitors extends the range to about 50 days. Slow-release products vary widely in their release timing, but generally are released within about 14 to 50 days. Polymer-coated products vary widely, depending on quality and thickness of the coating. Products are sold with release timings ranging from 45 to 360 days. Unlike most agricultural applications that are tilled into the soil, most nitrogen applied to grass is surface applied. The hot temperatures at the surface can shorten the times of all of these ranges listed above. For example, research on polymer-coated urea showed that most of the nitrogen was released within 45 days – regardless of coating thickness. Thus, contrary to some claims, these do not last the entire season, especially in warmer climates with long growing seasons.
Each of these enhanced-efficiency fertilizers can minimize volatilization, denitrification, and leaching losses, because there is no large flush of nitrogen-containing chemicals at any one time. Thus, plants can take up the nitrogen, and less is lost to the environment. Their use is highly recommended.
Two suggested approaches to nitrogen management.
Liquidnitrogen fertilizer applied as a foliar spray or injected into the irrigation system provides excellent coverage with each plant receiving nitrogen. Foliar sprays have the potential to be extremely uniform. In contrast, no irrigation system is perfectly uniform – with some areas receiving two times more nitrogen, even with well-designed/maintained irrigation systems. Nevertheless, these applications can be highly effective. Application of 0.25 to 0.5 lb N/1,000 ft2needs to be made at least every month of active growth, with slightly higher rates prior to winter dormancy and less during summer months. Combining a slow release nitrogen source and/or an inhibitor can reduce the number of applications needed to two to four times per year. Divide the amount of nitrogen desired by the percent of nitrogen in the fertilizer to obtain the amount of actual fertilizer needed (eg. 0.5 lb N /1,000 ft2divided by 0.2 for a 20-0-0 fertilizer = 2.5 lb fertilizer /1,000 ft2).
Solidnitrogen fertilizer is often less costly and requires less sophisticated equipment than a liquid program. For grass surfaces, it is essential to use products with a high size guide number (SGN), which means that the granules are relatively small. A high SGN nitrogen fertilizer results in improved uniformity. However, even high SGN fertilizers don’t result in as good of uniformity as a liquid foliar spray – with some plants not receiving any nitrogen in the short term and others receiving a relatively high rate. This lack of uniformity tends to equalize over many applications, but a spotty appearance can occur – especially on newly seeded/sodded surfaces. Application of 1.0-1.5 lb N/1,000 ft2with a blend of ammonium sulfate (if sulfur is needed) or urea and polymer-coated urea (or similar) should be made early spring at greenup. At least 2/3 of the mix should be the polymer-coated urea. Then, twice this amount should be applied in the early fall. This can be split into a third application if desired, but research shows no advantage for three vs. two applications.
In general, higher rates of nitrogen are needed if clippings are not recycled in place. Relatively higher rates are also needed on sandy soils and in other scenarios when leaching losses below the root zone are more likely.
However, it is vital to avoid excess nitrogen, which causes excess shoot growth at the expense of root growth. This results in increased water and fertilizer costs, disease, and mowing costs.
It is also important to avoid getting trapped into thinking that blended fertilizers are essential. Soil testing should be used and be based on legitimate scientific studies for interpretation for phosphorus, potassium, and micronutrients.
One of the most important things we do in managing grass and other plants is to get the nitrogen right. Doing this pays big benefits.
Bryan G. Hopkins, Ph.D., CPSS, is a professor at Brigham Young University, Provo, Utah.


